Abstract
Background: APL is the most curable leukemia with survival >90% in large co-operative group studies. These excellent outcomes are not seen in elderly patients where early deaths (ED) in trials are 10-18%. Population based registry data shows that ED rate in elderly patients is even higher at 24-50%. The one year relative survival was 37% in patients >60 years in Swedish population data. We report the outcome of patients >60 years from our experience.
Methods: We performed a retrospective chart review with IRB approval of 138 patients treated at leukemia treatment hospitals in GA, SC and neighboring states. Newly diagnosed patients were treated using a simplified set of treatment guidelines along with expert support designed to decrease ED. There were no exclusion criteria in this prospective trial. Given the comorbidities in this "real world" elderly population, dose reductions were necessary. We documented the doses of ATRA, ATO, and idarubicin given to patients ≥60 at diagnosis, and then calculated the percentage dose reduction the patient received, if any. For our calculations, standard doses were 45mg/m2/day for ATRA, 0.15mg/kg/dose for ATO, and 12mg/m2/dose for idarubicin. The target dose reduction for patients' ≥60 in the second half of the study was 25mg/m2/day for ATRA and 0.075mg/kg/dose for ATO. In morbidly obese patients, the BSA was capped at 2.0 and dosing weight for ATO at 100 kg.
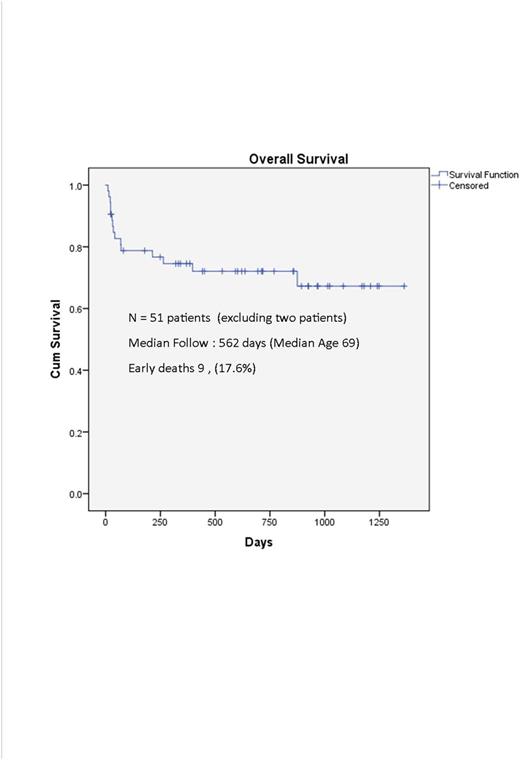
Results: Between 7/2013 and 4/2017, 138 patients were treated at 4 large leukemia centers and 30 community hospitals. Fifty-three patients (38.4%) were >60, with a median age of 69. Male to female ratio was 1:1. Median WBC was 2.3/mm3 (0.3-38); 93% were low risk. 25 (47%) were treated in our institution and 28 (53%) were managed in 18 other centers. Of this group, n=27 were ages 60-69, n=19 were 70-79, and n=7 were >80. 30 (56.6%) patients had scores of > 5 on the age adjusted Charlson Comorbid Index (range 2-12). Dose modifications were necessary given the high comorbidities which are not usually the norm in clinical trials due to exclusion criteria. A total of 53 patients (100%) received ATRA, 43 (81%) received ATO, and 7 (13%) received idarubicin (Ida). For ATRA, n=5 (9%) received an initial dose reduction of 10-24%, n=13 (24.5%) received reduction of 25-49% and n=7 (13%) received a reduction of ≥50%. For ATO, n=2 (4.7%) received a reduction of 10-24%, n=10 (27.9%) received a reduction of 25-49% and n=14 (32.6%) received a dose reduction of ≥50%. Only one patient received 50% dose reduced (Ida). The remaining patients received full doses. For six patients, the dose of ATRA (n=1), arsenic (n=5), and (Ida) (n=1) was not provided. Excluding two patients, one a Jehovah's Witness who refused transfusions and the other we were consulted 12 days after diagnosis with multi-organ failure, there were nine deaths during induction (17.6%). There were 6 late deaths from second cancer (n=1), relapse (n=1) and non APL related deaths (n=4). There were 3 relapses (2 high risk), none of whom received dose reductions and 1 refused consolidation. With a median follow up of 562 days the 1 year survival was 74.5% and overall survival was 70.6%.
Conclusions: One year relative survival in elderly APL patients is dismal with population based Swedish and SEER data showing rates <50%. Age and comorbid conditions dictate that a more personalized approach with dose reduction could result in better tolerance and improve outcomes in the elderly. A simplified treatment algorithm along with expert support has the potential to decrease induction mortality (17.6%) and improve population wide survival (1 year 74.5%).
Kota: Xcenda: Consultancy; Leukemia Lymphoma Society: Research Funding; Takeda Pharmaceuticals: Consultancy; Novartis: Consultancy; Incyte: Consultancy; Pfizer: Consultancy. Arellano: Cephalon Oncology: Research Funding. Heffner: ADC Therapeutics: Research Funding. Stuart: Novartis: Research Funding; Sunesis: Consultancy, Honoraria, Other: Travel Support, Research Funding; Astellas: Research Funding; ONO: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celator/Jazz: Research Funding; Amgen: Consultancy, Honoraria; Bayer: Research Funding; MedImmune: Research Funding; Seattle Genetics: Research Funding; Cantex: Research Funding; Incyte: Research Funding; Agios: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding. Grunwald: Amgen: Consultancy, Research Funding; Celgene: Consultancy; Alexion: Consultancy; Forma Therapeutics: Research Funding; Incyte: Consultancy, Research Funding; Pfizer: Consultancy; ARIAD: Consultancy; Cardinal Health: Consultancy; Janssen: Research Funding; Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal